- Contact 0870 350 7767
- |
- Advertise
Home > Creasefield Ltd > STORING AND HANDLING LITHIUM BATTERIES
STORING AND HANDLING LITHIUM BATTERIES
 News and PR from Creasefield Ltd - Published 16 September 2015
Taking good care of batteries, whether in use or while being charged or stored, is imperative to preserving longevity and performance levels.
News and PR from Creasefield Ltd - Published 16 September 2015
Taking good care of batteries, whether in use or while being charged or stored, is imperative to preserving longevity and performance levels.
Before attempting to handle cells or assemble battery packs, an industry professional or battery pack manufacturer should be familiar with handling and storage guidelines.
Primary and Secondary Lithium Cells
Owing to the differences between cell chemistries, guidelines across various categories can vary markedly between battery types. For example, primary lithium cells, made up of metallic lithium, can be stored for around a decade with minimal loss of capacity, whereas secondary, or lithium-ion, cells bleed voltage much more quickly.
The differences between primary and secondary lithium batteries have ramifications for the proper handling and storage of cells not just for their practical application.
Lithium-ion Storage
Generally speaking, primary batteries can be stored for longer than their secondary counterparts and can be more durable. However, as with all other types of battery, any lithium-based cell reacts to changes in temperature.
A temperature of around 20º C is ideal for both primary and secondary lithium chemistry to discharge, but for storage it is advisable to hold cells at even cooler temperatures in order to gain the maximum shelf life for each unit.
Storing lithium-ion cells at around 40% of their capacity has been shown to prolong their life and improve their overall performance.
Handling Batteries
Inappropriate handling of batteries, particularly at the assembly stage before cells have been combined into battery packs, is a major contributor towards the number one cause of battery failure: short circuits.
A battery pack manufacturer should ensure that work stations are free of conductive materials, which can short batteries if they come into contact with either terminal. Exposed cells should not come into contact with jewellery, metal tools and bare metal surfaces and should be transported in individual compartments in a non-conductive carrying tray.
Emergencies
Dealing with a volatile material such as lithium has led to the adoption of stringent safety measures within the battery industry.
The differences in chemistry between primary and secondary cells, with the presence of metallic lithium in the former, means non-rechargeable cells should have access to fire extinguishers and lith-X powder, which do not rely on water to put out fires.
Generally, compromised lithium cells can expel a range of hazardous chemicals, such as chlorine dioxide, sulphur dioxide and hydrogen gases, as well as acidic waste water and harmful electrolytes.
Primary and Secondary Lithium Cells
Owing to the differences between cell chemistries, guidelines across various categories can vary markedly between battery types. For example, primary lithium cells, made up of metallic lithium, can be stored for around a decade with minimal loss of capacity, whereas secondary, or lithium-ion, cells bleed voltage much more quickly.
The differences between primary and secondary lithium batteries have ramifications for the proper handling and storage of cells not just for their practical application.
Lithium-ion Storage
Generally speaking, primary batteries can be stored for longer than their secondary counterparts and can be more durable. However, as with all other types of battery, any lithium-based cell reacts to changes in temperature.
A temperature of around 20º C is ideal for both primary and secondary lithium chemistry to discharge, but for storage it is advisable to hold cells at even cooler temperatures in order to gain the maximum shelf life for each unit.
Storing lithium-ion cells at around 40% of their capacity has been shown to prolong their life and improve their overall performance.
Handling Batteries
Inappropriate handling of batteries, particularly at the assembly stage before cells have been combined into battery packs, is a major contributor towards the number one cause of battery failure: short circuits.
A battery pack manufacturer should ensure that work stations are free of conductive materials, which can short batteries if they come into contact with either terminal. Exposed cells should not come into contact with jewellery, metal tools and bare metal surfaces and should be transported in individual compartments in a non-conductive carrying tray.
Emergencies
Dealing with a volatile material such as lithium has led to the adoption of stringent safety measures within the battery industry.
The differences in chemistry between primary and secondary cells, with the presence of metallic lithium in the former, means non-rechargeable cells should have access to fire extinguishers and lith-X powder, which do not rely on water to put out fires.
Generally, compromised lithium cells can expel a range of hazardous chemicals, such as chlorine dioxide, sulphur dioxide and hydrogen gases, as well as acidic waste water and harmful electrolytes.
Other announcements from Creasefield Ltd
-
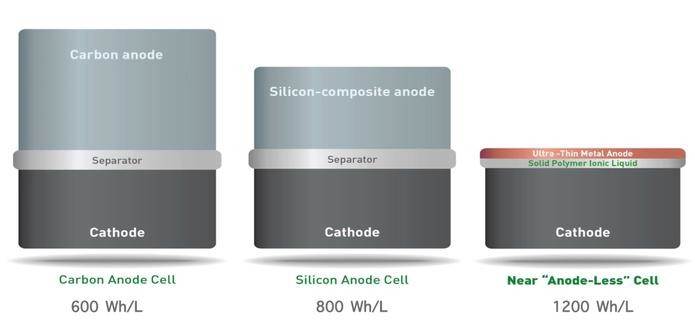
REINVENTING LITHIUM-ION BATTERY TECHNOLOGY
Massachusetts-based SolidEnergy Systems believe they have made a telling breakthrough in lithium-ion technology.
16 Sep 2015
-
SOURCE MEASURE UNITS (SMUS) AND BATTERY TESTING
The proliferation of lithium-ion batteries, with devices such as laptops, tablets and mobile phones all deriving power from them, has made battery testing a key area of research for every battery pack
16 Sep 2015